News
Structural Basis for Gating and Activation of RyR1
Posted by: Joachim Frank |
September 23, 2016 |
No comment
Our long-awaited Cell article on the activation and gating mechanism of the skeletal Ryanodine receptor RyR1 (a.k.a. calcium release channel) has finally appeared in Cell [1]. It is based on the highest-resolution maps obtained so far (up to 3.6 Å), which depict the channel in several states induced by the binding of calcium, ATP and caffeine, including a fully open state. The atomic structures determined from these density maps allowed us to infer the mechanisms of channel activation and gating, as sketched out in the graphical abstract below. Our article follows an earlier paper by Nieng Yan’s group reporting on a lower resolution map [2]. Interestingly, our publication coincides with Nieng Yan’s in Science [3] depicting the ryanodine receptor of mammalian heart, RyR2, at 4Å resolution.
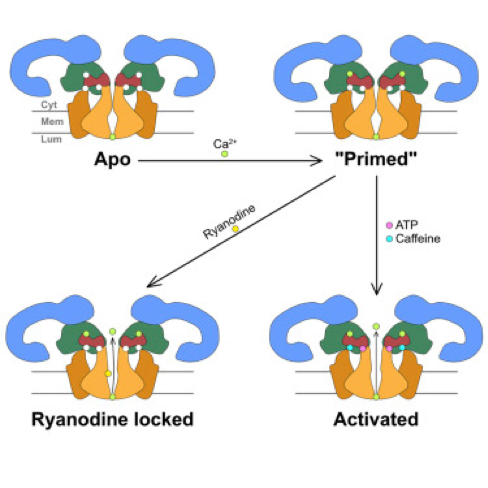
Summary
The type-1 ryanodine receptor (RyR1) is an intracellular calcium (Ca2+) release channel required for skeletal muscle contraction. Here, we present cryo-EM reconstructions of RyR1 in multiple functional states revealing the structural basis of channel gating and ligand-dependent activation. Binding sites for the channel activators Ca2+, ATP, and caffeine were identified at interdomain interfaces of the C-terminal domain. Either ATP or Ca2+ alone induces conformational changes in the cytoplasmic assembly (“priming”), without pore dilation. In contrast, in the presence of all three activating ligands, high-resolution reconstructions of open and closed states of RyR1 were obtained from the same sample, enabling analyses of conformational changes associated with gating. Gating involves global conformational changes in the cytosolic assembly accompanied by local changes in the transmembrane domain, which include bending of the S6 transmembrane segment and consequent pore dilation, displacement, and deformation of the S4-S5 linker and conformational changes in the pseudo-voltage-sensor domain.
[1] Amédée des Georges, Oliver B. Clarke, Ran Zalk, Qi Yuan, Kendall J. Condon, Robert A. Grassucci, Wayne A. Hendrickson , Andrew R. Marks, and Joachim Frank (2016). Structural Basis for Gating and Activation of RyR1. Cell 167, 145-157. http://authors.elsevier.com/a/1Tl-fL7PXN4Us
[2] Xiao-Chen Bai, Zhen Yan, Jianping Wu, Zhangqiang Li, and Nieng Yan (2016). The central domain of RyR1 is the transducer for long-range allosteric gating of channel opening. Cell Research 26, 995–1006. doi:10.1038/cr.2016.89.
[3] Wei Peng, Huaizong Shen, Jianping Wu, Wenting Guo, Xiaojing Pan, Ruiwu Wang, S. R. Wayne Chen, and Nieng Yan (2016). Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2.
Science, DOI: 10.1126/science.aah5324.



