Research activities
My group studies translation, the process by which the genetic message carried by mRNA is translated into a polypeptide chain, which then folds into a working protein. The primary tool for these studies is visualization by cryo-electron microscopy (cryo-EM) combined with single-particle reconstruction, a method of structure research developed in my lab. Molecules are quickly frozen so that they are trapped in a close-to-native state in a thin (~1,000 Å) layer of vitreous ice.
In contrast to x-ray crystallography, which requires highly ordered crystals, cryo-EM and single-particle reconstruction can produce a three-dimensional density map from freestanding macromolecules. This is done by combining tens of thousands of images showing different views of the macromolecule that exists in as many copies in the sample. This powerful method makes it possible to analyze the motions and dynamic changes of “molecular machines” (assemblies of dynamically interacting molecules that perform one of the cell’s functions, such as transcription or translation) during their functional cycle. If all assemblies are present in the same conformational state, then the density map produced by three-dimensional reconstruction can be interpreted as a meaningful “3D snapshot” of the macromolecular machine along its dynamic course. Since the resolution of such density maps is currently limited to 7–12 Å, sophisticated methods of docking and fitting are required to interpret each map in terms of the underlying atomic structures obtained by x-ray crystallography.
We have made significant progress in two aspects of the technology, both leading to advances in the elucidation of translation. First, we have advanced the methodology for sorting images of heterogeneous molecules into homogeneous subpopulations. Second, we have improved the cryo-EM resolution of a particularly stable ribosomal complex to 6.7 Å.
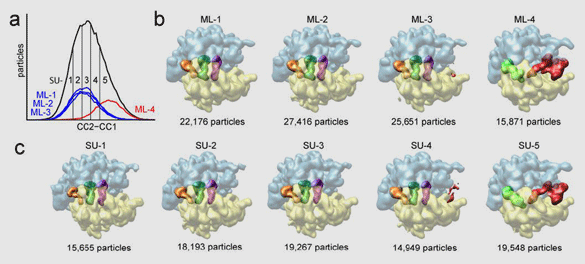
Often the sample contains the molecular machine in two or more different conformational or binding states, despite efforts of biochemical purification. These projection images must be sorted by conformation, which is difficult because conformational and orientational variability are commingled in the experimental data set. We have recently collaborated with the group of José María Carazo (Centro Nacional de Biotecnología, Madrid) to develop a powerful classification method based on maximum likelihood. In contrast to conventional supervised classification techniques used in single-particle reconstruction, which require the knowledge of reference structures, this new technique requires a minimum of prior knowledge (Figure 1).
Figure 1: Maximum-likelihood classification (ML) method, applied to a data set of 90,000 ribosome images (in collaboration with José María Carazo [CNB, Madrid]). The ribosome exists in two different conformations (+/– EF-G) and random views. Conventional “supervised” classification (SU; a, c) relies on a comparison of the noisy images with known references. The new maximum-likelihood method is able to perform the same classification (b) in the absence of prior knowledge. The reconstructions show the ribosome (30S subunit in yellow, 50S in blue) in a top view, partially transparent to reveal the positions of the tRNAs (A, P, and E in pink, green, and orange). Reconstructions at the far right show EF-G (red) and P/E hybrid tRNA (green).
From Scheres, S.H.W., Gao, H., Valle, M., Herman, G.T., Eggermont, P.P.B., Frank, J., and Carazo, J.M. 2007. Nature Methods 4:27–29.
The 6.7-Å map of the Escherichia coli ribosome is shown in Figure 2. The complex visualized here is “frozen” in a state after aminoacyl-tRNA has entered the ribosome in complex with elongation factor Tu (EF-Tu) and GTP, and GTP has been hydrolyzed. The antibiotic kirromycin was used to stall the complex in the GDP state. The map shows an extraordinary degree of molecular detail directly comparable to the x-ray structures of the ribosome and EF-Tu. At some places along the A-form rRNA helices, phosphorus atoms are actually visible as “bumps.” Both α-helices and β-sheets can be identified by appearance and by reference to the x-ray structures. The new map allows us to characterize the interactions between EF-Tu, GDP, and the ribosomal contact sites of the factor (notably the sarcin-ricin loop, which is involved in triggering the GTPase activity of the factor).
Figure 2: Cryo-EM reconstruction (at 6.7 Å) of the E. coli ribosome complex with aminoacyl-tRNA•EF-Tu•GDP (ternary complex) bound in the presence of the antibiotic kirromycin. EF-Tu is shown in red. Portions of the aa-tRNA are seen in pink. Parts of P- and E-site tRNA are seen in green and orange, respectively.
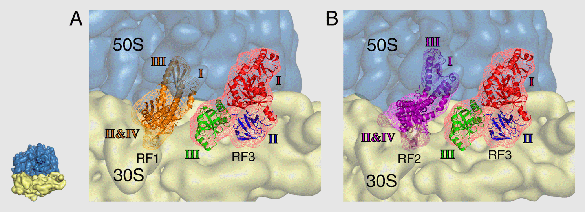
Our lab, in collaboration with groups in Uppsala and Singapore, has achieved a fuller understanding of translation by studying the termination process in prokaryotes. Normally, during the elongation phase of protein synthesis, sense codons move successively into the decoding center and are matched with cognate tRNAs. This process is terminated when a stop codon moves into the decoding center. Upon encountering a stop codon, ribosomal class-I release factors 1 or 2 (RF1/RF2) bind to the ribosome and cleave off the peptide from the P-site tRNA. Our group previously investigated these interactions between RF1 or RF2 and the ribosome. Most recently we addressed the question of the mechanism by which RF1 or RF2 is forced to depart from the ribosome. It was known that these release factors are ejected following the interaction of class-II RF3 with the ribosome, but the structural details of this process were not well understood. RF3 is a GTPase that binds to the ribosome at the same ribosomal site as EF-Tu and EF-G.
We have solved this question by a cryo-EM reconstruction of RF3 bound to the E. coli ribosome in the presence of GDPNP, a nonhydrolyzable analog of GTP. Furthermore, Haiwei Song’s group (Institute of Molecular and Cell Biology, Singapore) solved the structure of RF3 by x-ray crystallography, and Mans Ehrenberg and Suparna Sanyal (both at Uppsala University) performed complementary mutation studies. Our joint results (now published in Cell) indicate that RF3 does not displace RF1/RF2 via a steric clash, as previously concluded by another group based on a study with much lower resolution, but through the induction of a conformational change in the ribosome that results in a destabilization of RF3 binding (Figure 3).
Figure 3: Interaction of RF3 (red mesh) with the ribosome as shown by cryo-EM. The positions of RF1 (orange) and RF2 (pink) shown on the map indicate that there is no contact between either molecule and RF3. The conclusion from these findings is that RF3 acts indirectly, by changing the conformation of the ribosome, in bringing about the ejection of RF1/RF2.
From Gao, H., Zhou, Z., Rawat, U., Huang, C., Bouakaz, L., Wang, C., Cheng, Z., Liu, Y., Zavialov, A., Gursky, R., Sanyal, S., Ehrenberg, M., Frank, J., and Song, H. 2006. Cell 129:929–941. © 2007 Elsevier Inc.
Thus our study of termination and recycling, initiated in 2000 with Ehrenberg’s group, has explored every major step of the process: binding of RF1/RF2 and cleavage of the peptide (study with HHMI associate Urmila Rawat); RF3 interaction with the ribosome to effect the ejection of RF1/RF2 (study with HHMI associates Haixiao Gao and Urmila Rawat); and RRF and EF-G interaction with the post-termination complex (previous studies with Rajendra Agrawal and HHMI associate Ning Gao). We are currently engaged in a similar exploration of the termination process for eukaryotes.
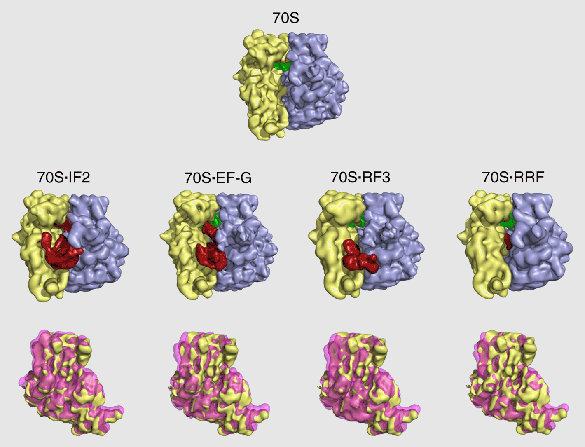
In the course of studying the binding interactions by RF3 and other factors with the ribosome, we discovered a fascinating detail that may hold a clue to the way the ribosome works and how it might have worked in the RNA world: binding of all factors, with the exception of EF-Tu, induces the ratchet motion, a counterclockwise rotation of the small subunit relative to the large subunit (discovered by my group in 2000 for the case of EF-G). The list of factors includes IF2, EF-G, RF3, and RRF (Figure 4). We found earlier in collaboration with Charles Brooks (Scripps Research Institute) that the ratchet motion is a natural consequence of the ribosome’s architecture, as it is a principal mode of the structure’s dynamic response to a mechanical perturbation. Our new findings imply that the ribosome performs several unrelated tasks by channeling energy from the thermal environment to achieve a conformational change that is productive for the respective task.
Figure 4: Ratchet motion in the E. coli ribosome induced by the binding of several factors. Upper panel: normal conformation of the ribosome. Middle panel: the various factors bound to the ribosome, inducing ratcheting. Lower panel: superimposition of small subunit in both conformations. RRF is not a GTPase, and IF2, EF-G, and RF3 are all bound in the presence of GDPNP, so the ratchet motion does not require GTP hydrolysis.
From Frank, J., Gao, H., Sengupta, J., Gao, N., and Taylor, D.J. (2007). The process of mRNA-tRNA translocation. Proceedings of the National Academy of Sciences U S A 104, 19671-19678..
The discovery that the ribosome has bistable properties intrinsic to its architecture and necessary for a variety of its functions could provide us with a glimpse of the way an “ur-ribosome” may have worked in the postulated RNA world to generate the first helper proteins.
This work was supported, in part, by grants from the National Science Foundation and the National Institutes of Health.